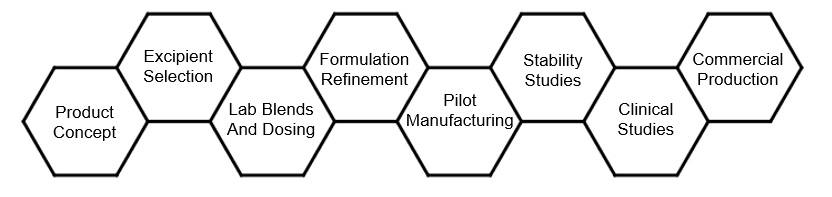
Formulation Development
Our team provides experience in the fine chemical, pharma and biotech industries and can help you at any stage in your development process. Our style is highly collaborative, emphasizing regular contact, open communication and full disclosure of progress – both accomplishments and roadblocks/issues, to enable rapid decision making and project success.
De-formulation Studies
- Literature/IP review
- API availability review
- Dissolution studies
- Blend compatibility (API + excipient)
Formulation of Solid Dosage
- API and excipient selection and testing
- Pilot scale blend, tablet, capsule suppository etc. manufacturing
- Small scale engineering runs to determine process validation parameters
- Accelerated and normal stability protocol design and execution
Analytical Method Development
- HPLC, GC, wet bench testing
- Parallel development: analytical methods designed with products
- Method validation and transfer to client
- Clean-out protocols and methods
- Stability and forced degradation protocols